The Investigation of the mechanism pizolits formation enables to develop a method and make theoretical generalizations, using which it is possible to calculate the temperatures under which the rings of pizolits had been formed. The pizolits are formed by the destruction of water- solved calcium hydrocarbonat, according to the following reaction:
Ca(HCO3)2 = CaCO3 + H2O + CO2 (1)
The resulting CaCO3 is the material, from which the pizolits develop.
The increase of the portion of CO2 in water presses the reaction (1) and forces the reverse reaction to begin:
CaCO3 + H2O + CO2 = Ca(HCO3)2 (2)
The Force Mass rule for heterogen system starts to affect the process. It establishes equilibrium between (1) and (2):
Ca(HCO3)2 = CaCO3 + H2O + CO2.
It means that at any given period of time a particular quantity of Ca(HCO3)2 divorces from the solution and turns into insoluble CaCO3, while a particular amount of CaCO3 dissolves and turns into Ca(HCO3)2. At the moment the establishment of the equilibrium the rates of straight and opposite reactions are equal:
V1 = V2 (3)
The formula for the rate of the first reaction is:
V1 = K1 .[Ca(HCO3)2]
The formula for the rate of the second reaction is:
V2 = K"[CaCO3].[H2O].[CO2]
As the concentrations of CaCO3 and H2O are constants, we well use K`2
K" [CaCO3] [H2 O] = K`2 .
Then the formula for the rate of the second reaction will be:
V2 = K`2[CO2] Which means that the rate of (2) reaction depends only from the concentration water - solved CO2. The variations of the quantity of water -solved CO2 are small: Therefore
Kí2[CO2] » const = K2 . Then the rate of reaction (2) reaction well be:
V 2 = K2 The constant of the rate of reaction is expressed by the following formula [22],[24]:
K = K 0.exp (-E/RT) where E- is the activation energy of reaction, K- is the preexponential multiplier, which is a constant number for this reaction, T- is the temperature, under in which the reaction take place.
After introducing the corresponding values the formula (3) will be as following:
K 0(1).exp(-E1/RT).[Ca(HCO3)]2 = K0(2).exp(-E2/RT) (4) The concentration of Ca(HCO3 )2 in the water is determined by the difference between the Ca(HCO3)2 confirmed in the water before the development of pizolit and the sediment of CaCO3, which developed from Ca(HCO3)2:
[CaCO3] = [Ca(HCO3 )]2 - P
equilibrium initial sediment For example, the quantities of ions contained in water of first spring of Devilís bridge, can be estimated from the Table 9 as followings (g. ion/ liter) :
(Na+ + K+)-3,2.10-3, Mg+2 -1,3.10-3, Ca+2 -1,2.10-2 Cl-1 -2,8.10-3, SO4 -2 -6,2.10-4, HCO3- -2,4.10-2. As we can see, the principal ions are Ca+2 and HCO3 - and they are contained in 1:2 proportion. It means that actually we have solution of Ca(HCO3 )2 with concentration of 1,2.10-2 moll / liter. The concentrations of the other salts is from 10 to 100 times smaller. The volume of CaCO3 sediment which divides from one liter of water under Devil`s bridge, approximately equals to 4.10-2 cm3 . The corresponding weight is:
P = 4.10-2 .d,
where d is the density of calcite (approximate 3 g / cm 3).
P» 4 .10-2 .3 » 0,1 g CaCO3 , or 1.10-3 moll / liter. So from each litre containing 1,2.10-2 moll / litre of Ca(HCO3 )2 1.10-3 moll divides in the form of carbonate, which is only 10% of the salt, contained in the solution. Such a low rate of production is caused by the free CO2 contained in the water of the spring. It influences reaction (1). Under 200 C the dissolubility of CO2 is 1,7 gram. This is the exact amount contained in the water first spring (1,7 gram in 20 OC). Therefore, for any particular cave the initial amount of Ca(HCO3)2 can be considered to be constant. A:
[Ca(HCO3)2]= A-P
equilibrium After introducing the equilibrium value of Ca(HCO3)2 concentration into [4]:
K0(1).exp.(-E1/RT.) (A-P) = K0(2) exp.(-E2/RT.) The fact that Ca(HCO3)2 spontaneously divides from the solution means that its energy of the activation of decay E1, is much smaller, than the energy of activation of the reverse reaction E2. That is E1 << E2, or E1 - E2 = - E2 . We can introduce the mentioned to the formula:
(A- P) = K0(2).exp(-E2/RT)/K0(1) And then write in the form of natural logarithm:
ln(A - P) = ln [K0(2) /K0(1)]- E2/RT K0(2) /K0(1) is a constant value. To simplify the estimates we can resume that the preexponential multipliers of the both reactions are equal: K0(1) = K0(2) .
In that case:
ln[K0(2) /K0(1)]= ln 1 = 0 The simplified equation will have the following form :
ln(A - P) = -E2/RT
T = -E2/ln(A-P).1/R (5) (5) correlates the temperature of the development of the sediment and the sediment itself.
To make practical use of formula (5) it in necessary to estimate E2. Lets install the data obtained under the Devils bridge for 20 OC (293 K):
A = 1,2.10-2 moll / litre , P = 1.10-3 moll / litre.
293 = - E2 / ln (12.10-3 - 1.10-3 ).1/2 = - E2/ ln 11.10-3.1/2
E2 = 2.(- 293). ln 11.10-3 = -2. 293.(- 4,5)
E = 2457 kal / mol = 10270,26 Joul/mol Lets introduce the value of E2 to formula (5):
T = - 1228,5/ ln (A- P). (6) As it is evident from (6), with the decrease of temperature P grows, which meet that the lower is the temperature of the cave, the more sediment is seized by CaCO3 . It means that in the winters a stouter layer develops on pizolits than in the summers. The above mentioned proves that the opinion, according to which the pizolit circles are correlated to the annual changes of temperature, is correct.
Formula (6) opens a field of new opportunities. Knowing A, which is a constant value for a particular cave, it is possible to calculate the temperature, under which any penticular ring of pizolit was formed. To do that it Is enough to measure P, which is the portion of CaCO3 contained in the given ring and expressed in g.mol/liter. Under the ideal conditions, when the center of crystalization is located in the center of pizolit, the portion of the layer of the sediment is possible to determine as the difference between the weights of two spheres, which are located in one another (Figure 26):
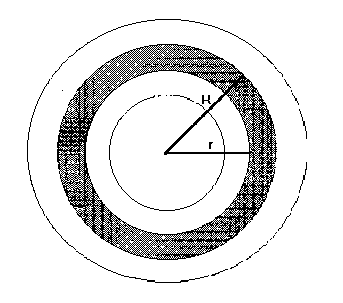
Figure 26. The cut of pizolit.
P = 4 / 3.p .R3 .d - 4 / 3.p .r3 .d = 4 /3.p .d( R3- r3 ) Where d is the density of pizolit (in gram). Letís introduce the above expression in (6):
T = - 1228,5 / ln[A - 4 /3.p .d ( R3 - r3 )] Whit reference to the different coloring of the pizolit rings, it must be explained not by the presence of mechanical alloys, as it is suggested in same works, but by different dissolution of salts. Spectral analysis showed, that pizolits contains for example, the following microelements[25]:
Si ~0,1%; Al ~0,0001-1%; Mg ~0,1%; Mn ~0,001-0,1%; Ti ~0,03-0,06%; Zr ~0,001-0,1%.
And of these microelements only the dissoluble salts of Mn and Ti are colored. Only the colored salts are the cause of the different coloring of the pizolits.
Using the formula (6) it in also possible to estimate the temperatures, under which the rings of stalactites were formed. In the case of stalactites the thickness of any layer can be determined as the difference of the volumes of two cones laated in one another ( Figure 27 ).
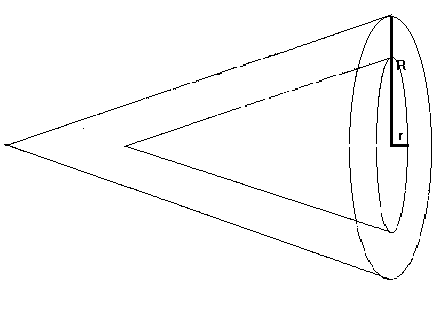
Figure 27. The cut of stalactite.
P = 1 / 3.p .R2 h.d- 1 / 3.p .r. 2h..d = 1 / 3.p . h .d.( R2- r2 ) h is approximately equal to the height of the stalactite.
By introducing the above equation to formula (6):
T = - 1228,5 / ln (A - 1 / 3.p .h.d ( R2-r2 ) (7)
Having the radius of any two consequent rings of a stalactite and using the formula (7) it is possible to estimate the temperature, under which the external ring was developed.