At present, some methods of minerals artificial obtaining are know[66]. They demand special equipments, high pressures and they are rather laborious. In this article in principle the new method of minerals origin is described. It doesn’t demand either expensive equipments, or high pressures, or complicated technology.
It is know, that existence of atoms and molecules of any solid substance in the gas phase is limited by the pressure of saturated vapours of the substance in the temperature given. In the number of solid substances with vapours low pressure metals oxides, salts, carbides and others meet one’s eyes especially. Pressures of saturated vapors of many substances of this kind are not measured up to the present ( ZnO, CuO, MoO, MnO2, CaCO3 and others ) [63]. Pressures of saturated vapours of certain among mentioned combinations are clarified only in a certain temperature sphere and are calculated by the following formula [63]:
Lg P =A - B/T
P-is the pressure of saturated vapours, T-is the temperature ( K ), A and B are the individual ratios.
Values of saturated vapours pressures of a number of solid substances in different temperatures are calculated with the help of the formula and are given in Figure 62.
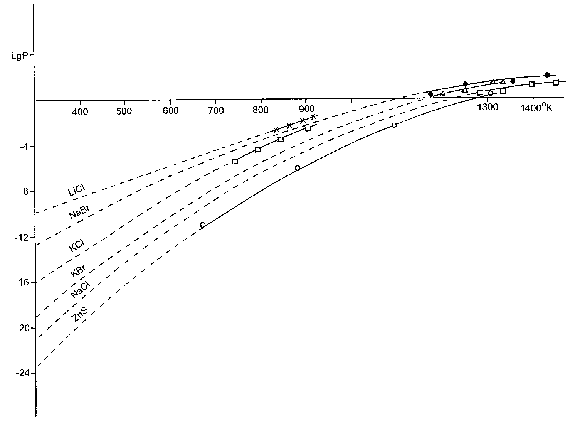
Figure 62. A number of solid substances saturated by temperature of vapors pressure dependence.
As it is seen from Figure 62, even in 1000 - 1200 OC the pressure of saturated vapours of those substances is hardly 101-102 tor. In normal conditions under the pressure one atmosphere and in ~0 OC those values reach almost the degree of the space vacuum. For example, ZnS vapours pressure in the room temperature forms only 10-24 ( Figure 62 ) tor, or only several molecules in 10 meters cubic. This is so slight value, that it is difficult to comprehend with physical-chemical ideas how diverse sulfides, oxides, salts and other combinations, which have the pressure of trivial vapours lift from the bowels of the earth and reaching the earth’s surface they turn into minerals.
To understand the phenomenon of minerals origin of this kind helps the new phenomenon discovered by us[56]. It leads to the experimental acknowledgment of a number of hypothetical phenomena and can find the employment in nature for clarification of the phenomenon of minerals spreading.
As it was said the phenomenon of transference of the solid substance to gas phase is not limited only by halogenids. Future researches showd [64] that that kind of property displays also oxides of metals and sulfides and with means of choice of the sphere corresponding to the pressure and the temperature we managed even sulfates [64], including also on copper sulfate what wasn't managed to observe in the work [56].
Kinetic and thermodynamical measuring confirmed that the transference of crystalline substances to gas phase is limited by the origin of new combinations. We must underline that the substance in clean state dosn`t transfer to the gas phase. On the surface, a combination with an unknown for the present structure ( X ) appears from interaction which take place between H2O2 and the solid substance, which transfers to the gas phase and displays perceptible stability. From its future decomposition separates staring crystalline substance: salt or oxide.
The substance described by general lines is imagined by the following way:
H2O2 + N ® hard substance ® X combination
X combination ® H2O2 + N
N + N ............ + N ® origin of a crystal
N - is the molecule of the substance which transfers to the volume. We must notice that the phenomenon of solid substances transference was researched in such a special temperature interval where the pressure of clean substance vapor's can be neglected ( see Figure 62 ).
At the presence of inert vehicles transference of substance such as H2O, CO2, O2 or Ar to the volume doesn't take place. This is a significant circumstance in nature from the point of view of the explanation of phenomenon process.
As it was said, the phenomenon of the transference of crystalline substance to the gas phase can supplement existing ideas regarding the mechanism of minerals transference.
The next circumstance, which is necessary to mention is the following: the pressure used by us were 10-1 - 10-2 tor, meanwhile the pressure reach hundred and thousand atmospheres in the cracks of the earth's crust. If it accept the specific weight of rocks 2,6 - 2,8 g./cm.-3 then the hydrostatic pressure will grow 2,6 - 2,8 atmosphere on one square centimeter with 10 meters depth each.
According to the Law of Influencing Masses the speed of the reaction depends on the product of substance concentration included in the reaction. So with the increase of the pressure the speed of the reaction increases. Hence, it isn't excluded that solid substances coming out to the volume in the cracks of the earth's crust takes place in lower temperature, than it is observed in the process of experiments.
Solid substances quantity coming out to the volume is also essential. During the experiments it formed 102 particles/cm.3 or 1018 particles/cm.3. Taking gases essential quantity lifting up from the bowels of the earth and the substance transference reactions proceeding long duration ( tens and hundred thousand, it is possible even million years ) into consideration, minerals considerable quantity origin by mentioned method is real.
Independently of the kind of the rock there exists directed flow of a gas substance from the bowels of the earth towards the earth's surface owing to the big gradient of pressure and temperature. It is especially appreciable in the zone of tectonic raptures [67].
H2O2 is also in the number of the gases moving up through the earth's cracks. Interacting with the wall of the cracks, it forms X combination with the crystalline substances containing on the wall, the crystalline substance transferees to gas phase and brings out to the earth's surface or to the layer near the surface. On the earth's surface X combination decomposing separates the initial chemical combination. Molecules of the separated substance crystallizing generate the corresponding mineral. Owing to the phenomenon of transfering of crystalline substance in over balance quantity to the gas ohase, the quantity of a solid substance in gas exceeds 105 and more times those quantities, which can be expected from solid « liquid balance condition.
The experimental material that we have gives the right to keep saying the fact of the existence of the unknown up to the present way of minerals transference at least for halids, sulfides, sulfates and oxides among minerals. We think that future researches will broaden this row.