The phenomenon which deserves attention was discovered in the process investigation of the phenomenon of heterogenous - radical decomposition of hydrogen peroxide.
Kinetic Method of Radicals Freezing and Accumulation in Combination with Electron - Paramagnetic - Radio - Spectrum - Meter ( EPR ) has found wide application for investigation of the mechanisms of slow proceeding compound gasophase processes [42]. Determination of nature of the accumulated radicals is one of the conditions of successful application of the mentioned method. At present there are a lot of information concerning spectrums of EPR multiatomic radicals, stabilized in a hard matrix.
It is necessary, nevertheless, to mention, that the conditions of radicals freezing influence the type of EPR spectrum. In some cases, it can bring to definite difficulties while identifying them. Specifically, it is shown, on the example of alkyl peroxide radicals, that the matrix environment and its temperature in other equal conditions influence the type of spectrum [45]. It would be natural to think, that the same can happen also in the case of HO2 radicals freezing. But this question wasn't subjected to special research.
HO2 radicals have EPR spectrum, which differs a little in main features from the spectrums of alkyl peroxide radicals. If also take into consideration, that in numerous gasophase chemical processes HO2 radicals can be formed together with other peroxide radicals, then necessity of the spectrum type determination under different conditions of their stabilization becomes obvious.
It is known, that during chemisorption of hydrogen peroxide on different hard surfaces, it decomposes to radicals, the part of which moves to the gas phase as HO2 radical [46]. In our conditions as the source of HO2 radicals - heterogeneous radical decomposition of hydrogen peroxide on the surface of molybdenum glass served. Choice of mentioned surface is conditioned by movement of more quantity of HO2 radicals to the volume in small degrees of H2O2 conversion. This circumstance is especially important in those experiments, where hydrogen peroxide is used as the stabilization substance and water quantity increases in a matrix is undesirable.
The device, used by as, is analogous to the described one [46]. The tube of molybdenum glass with 0,7 sm. diameter and 1 sm. length served as the reactor. The end with the shape of sticks of the same glass ( S / V » 60 sm.-1 ) was used with the goal of specific surface increase. Decomposition proceeded in stream conditions in temperature interval 200 - 400 OC, under peroxide pressure from 0,1 to3 tor. Time of mixture being in the reactor was regulated by valves and formed ~10-3 seconds. H2O2 pairs were chosen from ampoule, containing solid concentrated ( > 96 % ) hydrogen peroxide. In those experiments, where vapors pressure of hydrogen peroxide didn't exceed 0,1 tor, the whole gas flow after the reactor condensed on the freezing junction, placed in the sounding - beard of EPR spectrum meter. Under 0,2 tor pressures and more only the smaller part of the stream under P = 0,01 - 0,1 tor was sent to the freezing junction with the help of a crack. In all experiments the same crack ( d » 0,1 mm. ) was used, it is made of pirex glass and processed by aqueous solution of the hydrofluoric acid.
CO2, water and hydrogen peroxide were used as the matrix, which were added to the reacting mixture either directly to the reactionary zone, or after the crack. Temperature of the freezing junction, frozen by liquid nitrogen, was metered with the help of thin copper - constantan thermocouple ( d = 0,1 mm. ), which was put closely against the wall of the tip of the finger like shoot of Duar vessel. Measurements showed, that this temperature formed -183 OC. Presence of the thermocouple in the matrix could influence regularities of radicals accumulation. With the goal to prevent such feasible violation of freezing condition, the experiments concerning temperature measurement were made separately.
In the first series of the experiments we investigated influence of nature of stabilizing substance on the type of a spectrum [47]. In Figure 52 HO2 radicals spectrum is presented ( a ), received by interaction of hydrogen atoms ( they generated with the help of high quality type ) with oxygen, as well as in slow thermal reaction of hydrogen oxidation, under the first limit of combustion and stabilized in the inert matrix CO2.

Figure 52. Influence of the composition of a matrix the type of HO2 radicals spectrum: a - CO2 matrix, pressure after a crack Pc. = 0,3 tor; b - matrix CO2 and the reacting mixture. PCO2 : Pr.m. = 1 : 1; Pc. = 0,04 tor; c - matrix water and the reacting mixture. PH2O : Pr.m. = 4 : 1; Pc. = 0,1 tor.
The experiments showed, that if polar molecules are present in the matrix, then the type of the spectrum is a bit changed. Depending on the correlation of polar and non - polar molecules in the matrix, change of the signal form occurs differently.
Spectrum b is obtained during heterogeneous decomposition of hydrogen peroxide vapors in conditions, when the depth of conversion of H2O2 is not deep and hydrogen peroxide also is present in the matrix. Pressure of the mixture, which had already reacted, after the crack formed 4 - 10-2 tor. It isn't difficult to notice, that in such conditions disintegration intensity of A decreased, and disintegration of B almost disappeared. Decrease of CO2 in the matrix leads to the essential change of the spectrum, and CO2 absence in the matrix ( hydrogen peroxide of the reacting mixture served as the matrix ) leads to strong distortion of the spectrum.
The form of EPR spectrum of HO2 radicals is quite different, if we use water as the matrix. Spectrum c is obtained in the same conditions as b spectrum, with that difference, that in this case water concentration in the matrix exceeds five times hydrogen peroxide concentration. And regardless of the fact, that either water was provided to the reactor with a peroxide or after the crack, the same spectrum was registered. Obtained spectrum c remains invariable, if CO2 in concentrations, exceeding water concentration several times is added to the reach reacting mixture after the crack.
We must mention, that while irradiate water and hydrogen peroxide aqueous solution in frozen condition [48],[50] HO2 radicals are formed, EPR spectrum of which is analogous to spectrum, obtained by as.
In the next series of experiments influence of the matrix temperature on the type of EPR spectrum of HO2 radicals was investigated. With the goal to increase the matrix temperature the Duar vessel with double walls was used [51]. Gas pressure, in area between walls, separating the matrix from liquid nitrogen, formed ~5.10-3 tor, what ensured the matrix temperature increase to 20 OC.
In Figure 53 HO2 radicals spectrums are presented, which were frozen in two different temperatures -183 OC( a ) and -163 OC( b ) .

Figure 53. Influence of the temperature of a matrix ( Tm ) on the form of signal ( Pr.m. = 1,5 tor; Pc. = 2.10-2 tor; Tr. = 200 OC ). a - ( -183 OC ), b - ( -163 OC ).
Its easy to notice, that the matrix temperature increase leads to noticeable change of the signal form. Disintegration A almost disappears, and intensity of disintegration of C increases quite on the contrary.
It is interesting, that the matrix temperature increase allows also to observe definite influence of reactor temperature on the type of the spectrum. Influence of the reactor temperature on the type of the spectrum is observed in the case of colder matrix ( -183 OC ) too, but it occurs less distinctly. If to take in account, that during increase of the reactor temperature any changes of nature of occuring radicals don't proceed, then change of the spectrum type can be connected either with change of radicals quantity correlation to water quantity, existing in the matrix, or with the possibility of the temperature regime change of the matrix because of the reactor temperature change.
It is necessary to mention, that in high temperature of the matrix the type of the spectrum is more sensitive to any changes of the conditions of radicals obtaining and accumulation. The spectrums, presented in Figure 54, are good confirmation to what had been said. They are obtained in the matrix temperature -163 OC and in conditions, when in the reacting mixture water quantity 50 times exceeds peroxide quantity. The reactor temperature is 400 OC, pressure in the reactor is 3 tor, pressure after the crack is 5.10-2 tor, the reacting mixture serves as the matrix. Spectrums, presented in Figure 54 are obtained in the process of the same experiment but in different time of accumulation.
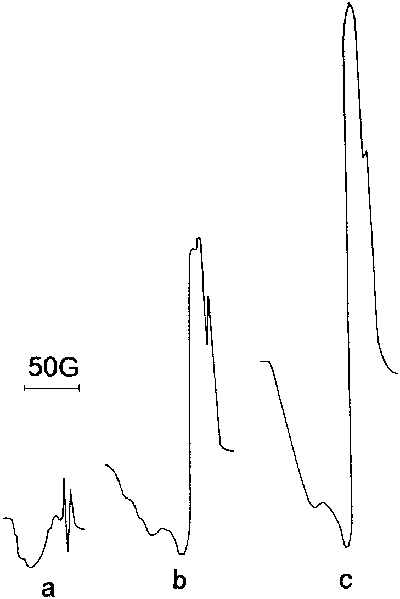
Figure 54. Signal form dependence on time of radicals accumulation in Tm. = -163 OC; a -2; b -10; c - 30 min.
It is easy to notice, that type of the spectrum changes powerfully with decrease of accumulation time. In short periods of time ( up to 2 minutes ) the type of the spectrum differs in the root way from the spectrums of peroxide radicals. Due to increase of accumulation time the spectrum turns gradually into that, which was obtained in the previous experiments, where water served as the matrix. Such change of the spectrum type in the process of radicals accumulation was observed only under high temperature of the matrix and large contents of water in the reacting mixture.
While investigating processes by radicals Freezing Method in real conditions it is often necessary to vary the value of the crack diameter, what brings to change of pressure after the crack under invariable pressure in the reactor. It would be interesting to know, whether this changes influence the type of the spectrum. It occurred, that in the same cases, really, pressure of the gas, which had reacted, after the crack can cause the spectrum form change, what is seen from Figure 55.
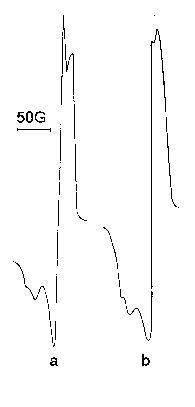
Figure 55. Signal form dependence on pressure after a crack ( Tm. = -183 OC, Tr. = 200 OC ); a -2,5.10-2, b - 9,0.10-3 tor.
Change of the signal form is more essential under low pressures. Such changes, obviously, are connected with determination of different temperatures of the matrix in the process of the stream freezing with different pressures.
So, obtained dates show, that the type of EPR spectrum is changed of various reasons, such as the matrix nature and temperature, pressure and speed of the stream in the freezing junction, correlation of radicals quantity to the quantity of existing in the matrix polar molecules and so on.