In order to elucidate, how every of pointed parameters influence the type of the EPR spectrum, detailed investigation of the matrix features is necessary in each concrete case. One of the main factors, determinating physical features of the matrix, is temperature undoubtedly. That is why investigation of the temperature regime of the matrix in the conditions of Kinetic Method of Radicals Freezing ( KMRF ) application is an important task [42].
We investigated regularites of the matrix temperature change in the process of such combinations freezing, which in KMRF conditions either is used as the matrix environment for radicals, or are present in the matrix as the components of the mixture, which had already reacted (CO2, H2O, H2O2) [52].
The experiments were done on the vacuum - stream glass device, the scheme of which is presented in Figure 56. The construction of freezing junction see in Figure 48.

Figure 56. The scheme of the device: 1 - graduated ampoule with hydrogen peroxide, 2 - reactor, 3 - tube, 4 - vessel with liquid nitrogen, 5 - thermocouple manometric lamp.
In Figure 57 temperature of matrix changes depending on time during CO2 freezing is shown.
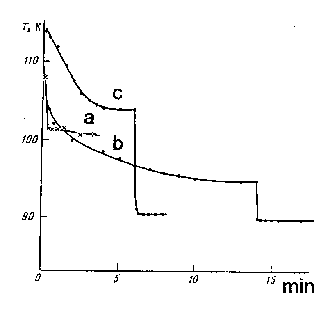
Figure 57. Temperature change dependence of freezing junction on CO2 accumulation time; a - without CO2 provision; b , c - CO2 stream pressure is 0,1 and 0,2 tor correspondingly.
The process of the external wall of the Duar vessel shoot from the moment of filling it with liquid nitrogen without CO2 ( curve a ). Provision temperature change is also presented there for comparison. Pressure in the freezing junction didn't exceed 5.10-2 tor. As it is seen from the Figure 57, during 30 sec. the external temperature lowers from room temperature up to ~100 K. If to create the stationary flow of carbon dioxide gas, then during CO2 accumulation the process of temperature decrease becomes slow. After stopping carbon dioxide provision, the matrix temperature lowers sharply up to 90 K and it remains steady later. The higher is the gas stream pressure, the more is difference of temperatures, so as to say, the more provision of heat by a gas stream. It is rather legal, that after stopping of CO2 provision, the same temperature is fixed in the matrix with various pressures.
In Figure 58 change of the matrix temperature in the process of water accumulation under various pressures of the stream is shown.
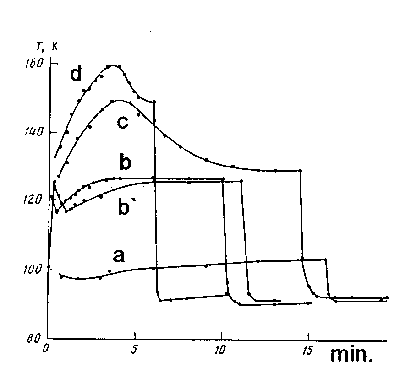
Figure 58. Temperature change dependence of freezing junction from time accumulation of water in different pressure of streame: 0,07 ( a ); 0,13 ( b, b` ); 0,13 ( c )( Tr. = 700 OK ); 0,2 ( d ) tor.
As it is seen, under water vapours pressure 0,07 tor ( curve a )during 16 min. temperature isn't subjected to special changes and only after stopping H2O provision, it lowers spasmodic to 10 - 12O and becomes equal to 90K. A kind of peculiar change is observed during pressure increase. In PH2O = 0,13 tor ( curve b )from the moment of the vessel being filled by liquid nitrogen, the matrix temperature lowers up to ~117K first and then it increases slowly and reaches ~125K meaning and it remains steady further. As soon as the provision of water stops temperature lowers to 35O and it forms 90K. The process of temperature change doesn't change, if the vessel is filled with liquid nitrogen beforehand, and only then, after determination of constant temperature of the freezing junction ( ~100K ), water vapours are provided. As it is seen, in this case, the curve of temperature change is displaced a bit to the side of long periods of accumulation time ( curve b` ).
In the real conditions of the reaction process the reacting mixture in the reactor is heating up to several hundred degrees. In KMFR conditions, pressure of reacted stream after the crack is lower than 1 tor, and its linear speed forms 5 - 10 m./sec. Possibility, that in such conditions, gas, passing the distance from the crack to the place of freezing ( l = 40 - 50 sm., d = 1 sm. ) doesn't manage to accept room temperature, isn't excluded. If it is so, then the reactor temperature change must lead to violation of the matrix temperature balanc, presented in Figure 58 ( curve c ) confirm the thought, which was expressed. It's interesting, that the reactor temperature rising influences the matrix the same way as pressure rising ( curve b ). Let's mention, that regardless of which moment of time water supply stops, the matrix temperature always lowers up to the lowest definite meaning ( 90 K ). This fact shows convincingly once more, that the matrix temperature regime change in the process of accumulation is connected to heat provision exclusively by gas frozen particles.
Comparison of the results, presented in Figure 57 and 58, shows, that the temperature regimes of CO2 and H2O matrixes differ greatly from each other. If in the case of CO2 the matrix temperature lowers gradually reaching a kind of constant meaning, then it is the other wayround for water. It is also interesting that under the same pressures of a stream the matrix temperature rising in the case of H2O is much more, than it takes place for CO2.
As we've already seen, regardless of whether CO2 is the matrix or H2O, after stopping of water provision under constant residual pressure the same temperature takes shape in the freezing junction. Taking different heatexchange of the matrix and environment, in these conditions changes of balance matrix temperature can be expected. In Figure 59 dependence of the temperature of frozen CO2, H2O and H2O2 on the pressure of the air, surrounding the matrix is presented.

Figure 59. Dependence of change of balance temperature of a matrix after stopping provision of CO2, H2O and H2O2 air pressure in the freezing junction: 1 -H2O ( snow ), 2 - H2O2 ( snow ), 3 - H2O2 (ice), 4 - CO2 ( snow ), 5 - H2O ( ice ).
In the case of water and concentrated hydrogen peroxide, their icy condition was also investigated. It's easy to notice, that in all cases the matrix temperature increases during the pressure rising regardless of wheather the matrix is in the shape of snow or ice. It's necessary, however, to mention, that the essential temperature change takes place under comparatively low pressures (P < 5.10-2 tor). Beginning with pressure 5.10-2 tor and higher up to the atmospheric, appreciable changes of the matrix temperature are not observed. The curves, received for icy conditions of water and hydrogen peroxide, are situated lower than the curves, received for their snow like conditions. Because of the matrix thick package in the case of ice, obviously heat giving to the cold wall of the Duar vessel is more effective, than heat provision by the gas, surrounding the matrix from outside. Such difference between heat giving and heat provision can lead to the decrease of the matrix balance temperature. Conclusion, that was done, is in good conformity with the results, received for CO2. The observations shown, that the CO2 matrix has thicker package, than snow like conditions of water and hydrogen peroxide. Obviously, that's why the curve of the CO2 matrix temperature change is situated lower than the others.
So, the results received by us show, than the matrix temperature can be changed after stopping of gases provision as well as in the freezing process. To our point of view, change of the matrix temperature regime right in the accumulation process determines its condition. Such big changes of temperature ( up to several tens degree ) can cause undoubtedly essential structural changes in the matrix and lead to the frozen radicals condition change by that. With more confidence we can confirm, that change of the type of the radicals EPR spectrums, caused by the matrix nature change, gas pressure after the crack, the reactor temperature and so on [47], in most cases is connected with the matrix temperature balance violation and, obviously, right on this stage of its formation. It is clear, that for radicals nature determination, stabilized in the hard matrix, it is necessary to show difference carefuless, while choosing the freezing conditions.