It is shown [54] by the Kinetic Method of Radicals Freezing [42], that hydrogen peroxide pairs on hard surfaces disintegrate into free radicals, the part of which moves to the gas phase and is fixed in the freezing junction with the shape of HO2 radical. Due to the results of H2O2 decomposition investigation on the surfaces of platinum and glasses in CO presence, the following conclusion [55], that HO2 radicals are formed by the scheme:
H2O2ads. = 2OHads
OHads + H2O2 = HO2ads + H2O
HO2ads ® HO2
It is necessary to elucidate whether hydrogen peroxide heterogeneous decomposition always is accompanied by the output of HO2 radicals to the volume for the true mechanism determination. With that goal in this work H2O2 decomposition was investigated on the surfaces, which differ from each other very much by their nature.
The researches showd [56], that on the steady surfaces, such as metals, carbides, sulfides, glasses and so on, decomposition is accompanied by radicals formation, the EPR spectrum of which is identical to HO2 radical spectrum, regardless of peroxide concentration, the reactor temperature, time of contact and so on.
Quite different situation is observed, if the reactor surface is covered with salts. Experiments, concerning peroxide decomposition on salts proceeded on the device, the scheme of which is presented in Figure 56. From the ampoule 1, which contains ( ~ 100% ) hydrogen peroxide, H2O2 pairs were let in through the reactionary zone with given surface, after what the whole gas flow was condensed in the freezing junction.
The reaction proceeded on the external surface of the tube, made of molybdenum glass ( d = 0,35 sm., I = 3 sm. ), twisted in a ring 2 ( d = 1 sm. ), the surface of which was covered by salts. The ring was heated with the help of nichrom spiral, fixed in the tube, what ensured local heating of the reactionary zone. The ring could be moved along the vertical axis of the tube 3 ( d = 1,5 sm. ) and fixed in different distances from the freezing junction 4.
Peroxide vapours pressure in the reactionary zone didn't exceed 0,1 tor and was metered with the help of thermocouple manometric lamp 5. Time of the stream passing through the ring ( reactionary zone ) formed 10-3 sec., and temperature formed 310 OC. Accumulation time formed 10 min., and the quantity of the radicals, accumulated in the matrix in these conditions, reached 1014 - 1015 particles.
The reactionary mixture ( H2O2, H2O ) served as the matrix for radicals stabilization in the freezing junction.
In Figure 60 EPR spectrums of radicals, accumulated in the freezing junction during H2O2 decomposition on the glass and salts KBr, NaCl, and KCl, are presented.
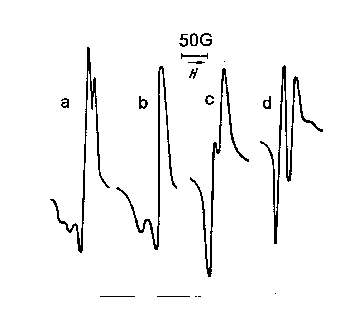
Figure 60. EPR spectrums, obtained during H2O2 decomposition on the surface of a glass ( a ), KBr (b), NaCl (c ), KCl ( d ). PH2O2 = 2,5.10-2 tor.
As it is seen, two first spectrums differ from each other a little, caving however, the main HO2 radicals spectrum outlines. Peroxide concentration change in the reactionary zone doesn't lead to special changes of these spectrums. However, the situation changes, when the reactor is covered by NaCl and KCl. In this case, the spectrums differ from each other and from the previous ones very much, though, decomposition conditions ( tk, PH2O2, T and etc. ) are the same everywhere. It's interesting to mention, that H2O2 concentration change in the case of NaCl and KCl influences the signal form, while taking the reactor away from the freezing junction ( from 1,5 to 20 sm. ) doesn't change essentially either quantity, or the frozen radicals spectrum.
This testifies the fact, that the registered signals refer to comparatively long living ( inactive radicals ). The spectrums 60a and 60b, received on NaCl and KCl in similar law peroxide concentrations ( 2,5 . 10-2 tor ) differ from each other very much, though the conversion degree is almost the same on both salts. It is curious, that only during peroxide concentration rising ( 0,1 tor ) the radicals spectrum, received on NaCl (60a) becomes identical to 60b spectrum.
It is necessary to pay attention to the fact of variety of registered EPR spectrums ( they are four, at least ). If to think, that each of these spectrums corresponds to a definite radical, then in the reaction of hydrogen peroxide heterogeneous decomposition it is difficult to imagine transfer from the surface to the gas phase with further concentration on the cold surface of such various radicals. Obviously, it is necessary to search the reason of such variety of signals in the change of nature of the matrix itself.
It is shown by special experiments, that freezing conditions and matrix environment influence the HO2 radicals spectrum. Depending on coreelation of peroxide in water and an inert diluter ( CO2 ) in the matrix, radicals spectrum changes a little. This fact shows, that such strong change of the spectrum in the case of H2O2 decomposition on salts is connected with existence of some other combinations in the matrix. It could be supposed, that either under H2O2 influence salt decomposes and its components are condensed in the matrix, or it is sublimated and reaches the cold surface, it leads to great changes of the matrix features.
We must mention, that in the work [57] during H2O2 decomposition on the glass surface, covered by KCl, 60d spectrum was obtained. Although, the authors didn't take the possibility of the matrix features changes into consideration because the salt existing in it and the spectrum was attributed to OH radicals by mistake.
So, the results, which were obtained, allow to conclude, that during hydrogen peroxide heterogeneous radical decomposition, regardless of nature of the active surface, decomposition temperature, contact time and H2O2 concentration, transfer the most in active in that system HO2 radicals from the surface to the gas phase in all cases. Depending on the freezing conditions, the matrix nature, etc., HO2 radicals spectrum can be changed very much. Moreover, that change is essential in those cases, when such strong polar substances like salt are present in the matrix.