The CO reaction with atomic hydrogen rises special interest for chemical kinetics:
H + CO + M ® HCO + M ( 9 )
The future reaction of HCO origin with oxygen rises significant interest for elucidation of aldehydes and hydrocarbons oxidization detailed mechanism. Dates, concerning this reaction, are contradictory [75]. In most of the works, conclusions about ways of interactions between HCO radical and oxygen was done based on indirect measurements. The reaction can proceed in three ways:
HCO + O2 ® HCO3 ( 10 )
® HO2 + CO ( 11 )
® OH + CO2 ( 12 )
It has been elucidated, that the ( 12 ) way isn't essential. The question of the possibility of other two ways is principal. The priority of ( 10 ) way is mentioned in a lot of works.
We investigated the interaction of HCO radical and O2 separately [75].
The experiments were done in low temperatures, in order to create such conditions, when radicals and, especially, supposed HCO3 radical decomposition is brought to the minimum. The reaction of hydrogen atoms and formaldehyde interaction served HCO radicals source:
H + CH2O ® H2 + HCO ( 13 )
Formaldehyde photolise was also used:
CH2O + h ® HCO + H ( 14 )
The scheme of the device is given in Figure 70.
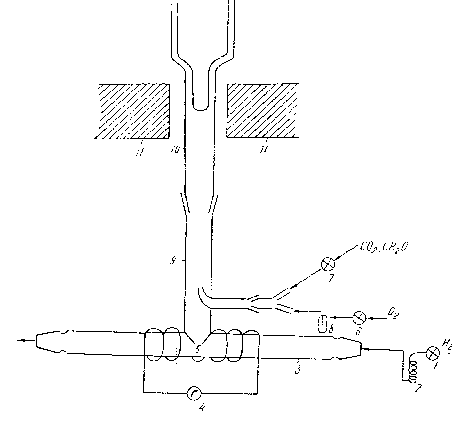
Figure 70. The scheme of the devastation wave.
Hydrogen under 4 tor pressure was cleaned beforehand, passing through the winding tube, which was sank in so in liquid nitrogen. Then in devastation tube, in hydrogen shield high frequency devastation is realized ( generator KV - 2m ). Plasma, containing atomic and molecular hydrogen, occurs. A small part of that gas mixture with the help of d = 1 mm diameter crack, through pump was sent to the reaction zone. Formaldehyde, oxygen and carbon dioxide gas were given to the reaction zone with the help of a pump from another crack ( d = 0,3 mm ). Formaldehyde was given for the realization of the ( 13 ) reaction, oxygen is for HCO radical future conversion proceeding by the reactions ( 10 - 12 ) and carbon dioxide gas, occurring for peroxide radicals freezing and hunting. In the reaction zone the pressure was 0,4 tor.
Tens of difference between the pressures of devastation and reaction zones excluded the diffusion of particles from the reaction zone towards the devastation zone. Generating peroxide radicals were hunted on the finger of Duar vessel, which was frozen by liquid nitrogen and it was put in resonator of Electron - Paramagnetic - Radio - Spectro - Meter ( EPR ). The Kinetic Method of Radicals Freezing was used [27].
Hydrogen atoms were in the reaction with CH2O due to the reaction ( 13 ). HCO radicals, which had occurred, could be in the reaction with oxygen. The pressures difference between devastation and reaction zones prohibited CH2O, O2 and CO2 diffusion to the reaction zone.
In conditions of the given experiment ( 0,4 tor )
H + O2 + M ® HO2 + M ( 15 )
reaction was excluded and HO2 radicals origin of H and O2 was possible only of Duar vessel cold walls.
In the first phase of the experiments the distance from the crack, providing hydrogen atoms to Duar vessel finger formed 21 sm. The experiments were done in absence of formaldehyde. HO2 radicals signal was registered, with the help of which we judged about H atoms origin. They were changed into HO2 on the cool finger of the Duar vessel.
The second phase of the experiments was done in formaldehyde presence. In order H atoms not to reach the cold edge of the Duar finger, we increased the distance from the crock to the edge of the Duar finger up to 53 sm., by adding the glass tube with d = 0,4 sm. and l = 32 sm. In that case EPR signal was absent, which testified about death of H atoms in the distance up to the Duar vessel edge.
Reaction atmosphere providing formaldehyde EPR signal was registered, which was identified as HO2 radical ( Figure 71a ).

Figure 71. HO2 radicalsEPR spectrum created with the help of hydrogen atoms ( a ) and EPR spectrum, generated of HO2 radicals from formaldehyde photolise ( b ).
Also formaldehyde and without formaldehyde radicals concentration were of the same kind ( ~1014 particles/sm3 ).
In the other phase of the experiments 20 % CH2O + 80 % air mixture photolise sensibilized with mercury vapors was used as HCO radicals source. It was fulfilled with the help of medium pressure lamp ( PRK -2 ). As insignificant part was stretched from the shield of air and formaldehyde mixture with the help of capillary and under the low pressure ( ~0,3 tor ) it moved towards the freezing junction through the quartz tube. After the capillary, mercury vapors were given and the mixture was subjected to photolise. It is known. CH2O photolise sensibilized by mercury vapors proceeds in two parallel reactions [13]:
CH2O + Hg* ® HCO + H
® H2 + CO
In order to take H atoms away and to investigate HCO + O2 reaction the distance from the point of photolise up to the Duar vessel was increased up to 70 sm. In that case the changed into the reaction zone and changing the distance up to the Duar vessel and it was identified as HO2 radical spectrum. The intensity of the signal formed ~1012 particles/sm3 ( Figure 8b ).
We didn't manage to register HCO radical signal. Obviously, the imprints of oxygen existing in formaldehyde were enough for fulfill the reaction ( 11 ).
So, HO2 radicals the same spectrum was both in devastation experiments and in photollise experiments. Not big difference of the frailest structure is explained by different concentrations of gathered radicals and the difference of the matrix. In the case of photolise, formaldehyde served as a matrix.
The results, which were received, testify, that even in the room temperature HCO radical reaction with oxygen proceeds quickly enough by ( 11 ) reaction, but not by ( 10 ) reaction.
Judging from the works [76],[78] , where values close to speed constant were received in the temperature big atmosphere for ( 11 ) reaction, we can conclude, that it proceeds without energy of activation.
So, HO2 radical generates as the result interaction, proceeding between HCO radical and oxygen.
Generated HO2, recombining on the walls of a cave or, in gas phase, taking hydrogen away from various organic combinations, generates hydrogen peroxide:
HO2 + RH ® H2O2 + R in air ( 16 )
HO2 + HO2 ® H2O2 + O2 on the wall of a cave ( 17 )
In this turn, generated the hydrogen peroxide, rises a number of special reactions, which generate unique conversions in cave atmosphere.