Chemical reactions are divided in two large groups: the reactions proceeding in a volume and the reactions proceeding on the surface division phases. Those reactions which proceed on two phases division surface, are called heterogeneous reactions. We separate a number of chemical processes proceeding in caves like reactions which have surface process. For example, the process of mumian medicinal resin origin [13]. In one of the caves situated in Northern Armenia, in Bndgraer ( literal translation - the cave of wild vegetables ) cave neighboring Akner village, Lori region we discovered the interesting accumulation of mumian. The slanting bottom of the cave is covered with manure of wild goats, which is a raw material for mumian origin. Though the condition for mumian origin on the bottom of the cave is the same everywhere, but the medicinal resin originated only in definite points (Figure 72).
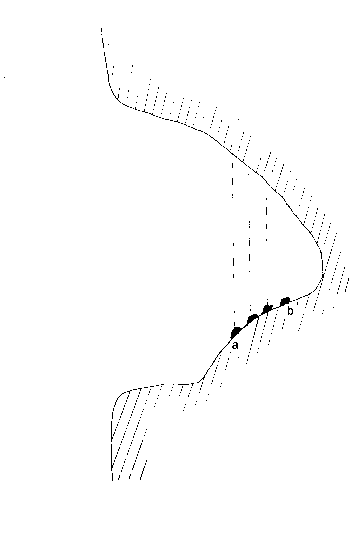
Figure 72. The section of Bndgraer cave ( Northern Armenia ). The places of basalt dust flow down from the ceiling of the cave are indicated by dotted line. On the sloping bottom of the cave, in ab part lifting up great lumps are the massifs of mumian.
Definite parts of the ceiling are comparatively easily weathered and the rock (basalt) dust flows constantly on the bottom from those points and it is mixed with manure, mumian origin proceeds.
In the parts where dust isnít mixed with the manure, its volume becomes smaller ( Figure 73, A curve ) and the manure is destroyed being weathered. On the contrary to it there, where basalt dust flowing from the ceiling is mixed with the manure, swelling of a raw material massif takes place and the raw material turns gradually into valuable natural medicine ( Figure 73, B curve ). These are those points, which are indicated with the look of great lumps lifting up from the bottom in Figure 72.
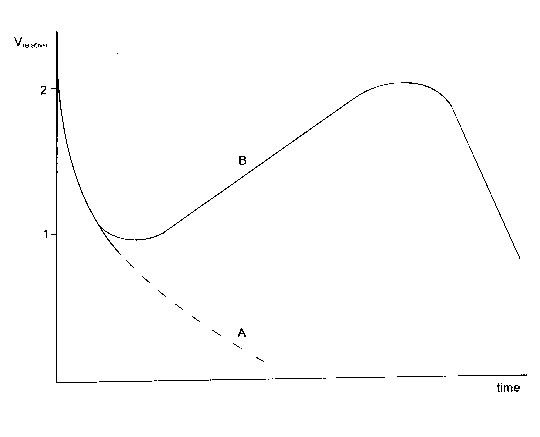
Figure 73. The relative alteration of the volume of wild animal's manure in the cave: A - without the influence of heterogeneous factor, B - with the existence of stone dust and mumian origin.
The example of mumian comments the necessity of taking surface factor into consideration in some reactions proceeding in caves.
Letís discuss another example: again in the Northern part of Armenia, in the region medium flow caves in Dzoraget river canyon we discovered natrium selitre NaNO3. It can be met in insignificant quantities in narrow cracks with 1 - 2 mm thickness protected from wind and rain and with the look, which has several square centimeters surface. With average account 1 gram NaNO3 contains on the surface ~1m2 of a rock. How the researches clarified, selitra was used by local inhabitants in the production of the black powder in ancient time.
In general, celitra ( NaNO3 )exists in cave there, where there are birdís droppings and light, but celitra, which is met in the volcanic caves cracks mentioned above, separates with its cleanliness and brightly expressed crystal structure. It obviously has the heterogeneous origin, because it is met in exclusively narrow cracks, which have developed surface and small volume. Probably, it origination in rock-gas heterogeneous division surface from nitrogen dioxide interaction coming out of the earthís crust cracks and aluminum-silicates containing natrium, being included in the composition of basalt.
If "X" reaction had homogenous process in a cave, crystal sediment presenting like the end-substance of the reaction had to originate proportionally in the whole volume, them the originated particles had to sit the bottom. Meanwhile, chemical combinations presented with the look of minerals are often divided in cracks. The evidence of it is famous in Armenia Achari volcanic cave [20]. The crack situated in depth of the cave gets gradually narrower and great crystals of aragonite ( CaCO3 ) are in the narrowest part of it ( Figure 74 ).

Figure 74. The plan of Achari cave ( Northern Armenia ). The place of crystals in the cave is shown by the arrow.
The existence of SiO2 in basalt and specifically in basalt caves pores can be explains by the great surface of pores which are in the rock. SiO2, which is met in bubbles of the walls, is in amorphous condition and it doesnít have expressed crystal structure [79],[81]. Even with the naked eye it isnít difficult to notice the following: SiO2 in basalt pores originated that time when the pores were already formed, so the lave was already frozen. SiO2 originated in the heterogeneous phase from the gas substances, which penetrated pores of still hot lave, which had got its final structure and it covered the internal surface of lump-bubble. As soon as SiO2 is in amorphous condition, so quick separation of SiO2 took place. This kind of quick separation can take place in the process of combustion of silans ( SiH4 and others ). The source of silans origin can be the interaction in the high temperature of silicon ( Si ) involving in the composition of lava and hydrogen diluted in lava.
Approaching the earthís surface, silans interacting with oxygen can oxidize instantaneously, separating SiO2 and water:
SiH4 + 2O2 = SiO2 + 2H2O.
That reaction has homogeneous - heterogeneous process and depends strictly on surface - volume correlation (S/V).
Letís observe the possibility of surface alteration of two main groups of caves: volcanic and karst caves. Letís discuss that case when a volcanic cave represents itself like a lump volume ( Figure 75 ).

Figure 75. Lava bubble-cave from Northern region of Republic of Armenia ( Dzoraget canyon ). A - the plan of the cave-bubble, B - section with aa1 flatness, C - section with bb1 flatness.
Lava bubbles - caves are like that. If the rock, which forms the cave walls, is homogeneous then its overall surface is defined by the formula:
S = 4 p r2 : 2.
Though there can be caves, where the walls can have small bubbles originated by volcanic gases. The sum of the surface of those bubbles leads essential changes in the surface of the cave and makes the role of heterogeneous reaction greater.
Lit`s count the greatest number of the bubles, which can be contained in lumplike cave walls with R = 2m diameter. The surface of cave - halflump is calculated by the following formula:
S = 4 p r2 : 2 =( 4 . 3,14 . 4 ) : 2 » 25 m2.
Letís accept that the walls are covered everywhere with small pores which have
r = 0,1 mm ( 1 . 10-4 m ) radius. Each pore represents itself as a halflump. The surface of circle of halflump will be:
S = p . r2 = 3,14 . ( 1.10-4 )2 » 3 . 10-8 m2.
The maximum number of small bubbles, situated on the surface of cave - lump, will be:
n = 25 : ( 3.10-8 ) » 1.109.
The common surface of one bubbles will be:
Si = 2p r2 = 2 . 3,14 . ( 1.10-4 )2 » 6.10-8.
The additional surface created by bubbles will make:
S1=n Si= 1.109 . 6.10-8 = 60 m2.
The total surface of the cave makes:
S+S1 = 25 + 60 = 85 m2.
In two caves, which have the same radius, one of which is in the solid rock, the other is porous, the total surface (bottom + walls + ceiling) differs several times, causing with that the increase of the role of the heterogeneous reactions.
In karst caves, diverse secondary chemical origins stalactites, stalagmites and others appear as the factors which make the surface bigger. If the karst cave is observed like homogeneous reactor, diverse chemical origins which are contained in it can be observed like ,,rods" thirsted in that reactor, which make the total surface of the cave bigger in effective way.
Stalagmites, which are called "cactus" ( Figure 76 ) create especially developed surface. "Cactuses" originate under the influence of water drops which were dripping from the ceiling and the in the second phase of the formation they are covered with ,,needles".

Figure 76. The usual stalagmite ( from the left ) and the stalagmite of " cactus " kind (from the right).
The surfaces of usual stalagmite and the ,,cactus" of the same sizes differ from each other. For clarity both can be observed as ideal truncated cone, for which the lateral surface
Slateral = p ( R + r ) l .
R and r - are the radiuses of two circles of the truncated cone, I - is the height of the cone. Usually stalagmites, reminding the truncated cone, their upper and under areas of the surfaces can be neglected. In the case:
S staiagmid » S lateral = p ( R + r ) l.
When stalagmite turns in to ,,cactus", the surface of its ,,needles" can exceed the surface of the stalagmite several times. For example, with I = 1m, R = 0,2m, r = 0,1m sires stalactite surface becomes from ~ 0,9m2 ~ 3m2.
In karst caves, crumbs of crystals create the surface developed with especially big sizes. For example, in Bears` cave of Yegheknadzor region ( Southern Armenia ) [20], N3 hall has approximately 5 . 5 . 1,5 m3 sizes. The total surface of the cave ( bottom, walls) makes ~ 80m2.
The sediment with ~ 0,3m3 volume is accumulated on the bottom of the hall. The deposit consists of the remains of calcite crystals. The diameter of the crystals is from
1 to 3 mm. Each crystal approximately can be accepted as a lump with r = 0,5 mm radius:
Si = 4 p r2 = 4 . 3,14 . ( 5.10-4 )2 » 3.10-6 m2.
Vi = ( 4p r3 ) : 3 = 4 . 3,14 . ( 5.10-4 )3 » 5.10-10 m2.
In the case of poky covering the number of crystals in volume will be:
n = 0,3 : ( 5 . 10-10 ) = 6.108.
The total surface created by the particles will make:
S = 6.108 . 3.10-6 = 1800 m2.
Meanwhile the surface of the hall of the cave without the crumbs of the crystals is 80m2 in all.
The halls off this kind are natural reactors, where the heterogeneous reactions go in intensive way. Future works, we think, will reveal new examples of heterogeneous reactions proceeding in the caves.