It is discussed the active role of caves in the ecological cleaning of gases, exiting from the crust of the World. It was showed, that the walls of caves, and also the clays, the stalactites and the stalagmites can be the natural reactors for bifunctional catalyze
The bifunctional catalysis is a new direction in the modern catalyze. One of the newest version of this direction is the method Spilover of hydrogen [82]. The word spilover is formed bythe words ,,spread,, and ,,over,,. The essence of the method is following: one of the components of reaction - hydrogen activates on the activecenters of catalyst. In that time receivethe adsorbedatoms of hydrogen whichs are very mobile and areeasy moved. They pass tensmillimeters way and practically spread on all surface and create best conditions for reaction. Passing on the other inert centers, which are in contact with the catalyst, they easy react with the molecules adsorbing on those [83]. Thisallows in the mildconditions, that is in the room temperature and atmospheric pressure the essential speed run such processes, the runs of which in other conditions demands big energy for activation, that is the high of temperature and the high of the pressures. Onthis way can be realizedprocesses of hydration, hydrogenation cracking plantand hydrogenation purify.
As the activators for Spilover of hydrogen reckon are considered the noble metals: Pt, Pd, Rh, Ru and the precious metals Cu, Co, Ni, Fe [84]. Good activators are also considered the following alloy of metals: ZnNi, ZnCo, ZnCu and the carbides of different metals: MemCn, where m and n are the numbers of metal and carbon atoms. As an takingof inert carrierserve SiO2, Al2O3, their mixture and the other substances.
Thehydrogen’s Spilover phenomenon allowssomedifficult reactions to run on the active surfaces easily and it can have large spreading in nature, if there are the conditions, when the not- high temperature of reaction surface is associated with the longtime of reaction (time of contact). The similar conditions are realized in the caves.
The two main rocks of the caves forming arena the volcanic and sedimental rocks,whereaccordingly volcanic and karstic caves are formed. In two cases the different metals: Cu, Feand many others enter in the structure of walls of caves. In that walls and in the clays, which arevery often met in the caves, always with thepresence of the oxides of Si and Al. They are those taking ofinert carrier , on which go the further conversions of hydrogen atoms, which are goingon the surface( see the reactions 1-8).
In the wall of volcanic cave by the elementary analysis we received the following average composition of microelements (see Figure 77) [86]. The emission- spectrographic method was used (flaming variant).
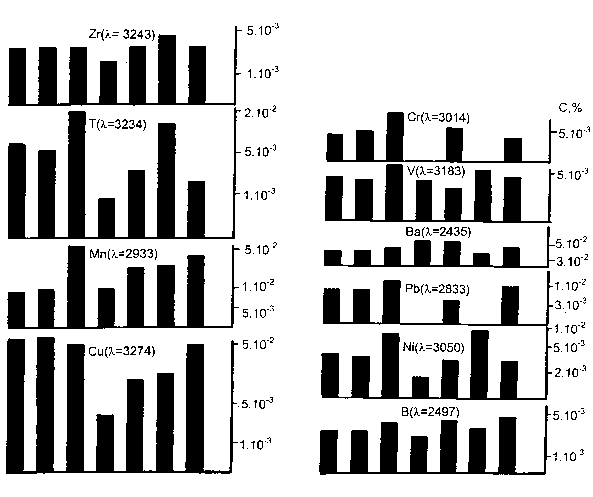
Figure 77. The microelementar composition of volcanic caves walls.
The average composition of the same basalt lavas in the form of oxides are the following [85]:
Table 12. The average composition of Lori's lavas.
SiO2TiO2Al2O3 Fe2O3FeO2 MnO CaOMgO Na2OK2OP2O3 H2O
49,37 1,38 18,174,825,940,159,454,933,75 1,520,090,26
This dates are near the dates of lava generally [85].
As we see by the Figure77 andthe Table 12, the walls of volcanic caves satisfy all the necessary conditionsfor Spilover of hydrogen.
It is the same as in the case karst caves [87]. In the Table 13 is broughtthe quantity of microelements in the walls of karst caves and in the different types of carbonic sediments of karst caves ( stalactites, stalagmites, crusts and others).
Table 13. The elements in the rocks and in the carbonic sediments of karst caves (n.10-3 %).
MnNiCo V Cr MoTi ZrCuZnGa LaYSr
limestone 2,6 0,08 00,23 0 0,08 3,1 0,69 1,3 0,80 1,2 0,2 18,5
sedim. 11,6tra. 0 tra.0 0,1 2,9 0,2 1,20 0 0,2 1,5 2,7
sedim. 212,40,60,044,91,10,08 228 5,52,0 0 0,10,8 1,02,5
rock87,65,9 1,4 11,98,20,3508 28,211, 3,92,02,22,6 1,6
The maintenance of general taking of inert carriersSiO2 and Al2O3 in the stalactites, stalagmites stone flowers and the other sediments of karst caves see on the Table 14 [87].
Table 3. The average composition of stalactites, stalagmites, stone flowers and the other sediments of karstic caves( % ).
SiAl Mg Ca FeMn Ti CuNiAg V NaK Sr Ba
~1~1~1 >>1 ~10-2~10-3~10-1 ~10-3 - ~10-3-~10-3 ~10-3 ~10-1 ~0-1
But the clays, which there are much in the caves mainly consist ofoxides of Si and Al: SiO2- 30-70%,Al2O3- 10-40%,H2O- 5-10% [88].
It was investigated also the crystalline structure of stalactites, stalagmites and the other carbonic sediments by microscope. It was observed, that they have avery developed surface. For example stone flower, which occupiesonly 4 cm2 surface under the ceiling of cave, is composed by ~ 40 branches, each of which has ~ 2 centimeter length and 0,075 centimeter thickness. The average area of one branch (Si ) will be:
Si = 2 . r.h= 2. 3,14. 0,075. 2 ~ 1cm2.
The surface of all branchs ( S ) will be:
S= Si. N= 1. 40= 40 cm2.
Every branch has much small thorns, the area of which is ~40 cm2. The common area of flower will be ~ 100 cm2.
The clays have more largearea: the average diameter of particles is smaller, than 1.10-3 cm. Even iftake, that the diameter of clay particles is equal 1.10-3 cm, inthat case in 1 cm3 of clay's will contain maximum 109 bells of clays. The area of one ball will be:
Si = 4.p .r2 = 4. 3,14. (5. 10-4)2 ~ 3. 10-6 cm.
The common area of all clay balls will be:
S = Si. N = 3. 10-6. 109 = 3. 103 cm2.
As the results of our experiments and the our calculations, and also the facts, whichare in literaturelead usto a conclusion, that the walls of all caves are the natural reactors for hydrogen's Spilover.
In the caves at the same type there are the activators of hydrogen- Fe, Cu and others and the taking of carrier- SiO2, Al2O3 and others This case leads us to ato conclusion, that the gases, which run out of World's crust are exposed to serious changes in the caves, arealsononestimate their role in the process of ecologicalcleaning of gases streaming to atmosphere.
Let's discuss several processes, which have the ecological interest. It is necessary to note, that in the gases, which run out fromWorld's crust there are the sulfuric gases SO2, SO3, CO, CO2, CH4, the high hydrocarbons [17],[52], the oxides of nitrogen, the vapor of water, and in the volcanic regions also H2, F2, Cl2 and others. [89].
There are the caves, where from the chinks of caves run out real gaseous fountains. For example CO2 (5,13-58,5% )+ N2( 46,5 -94,3% ) + CH4(0-0,62% ), or CH4( 94,9-97,5% ) +N2( 4- 3,7% ) [52]. With them can run the following reactions, where Z1- is the active center of activator and Zn- is the active center of of taking of inert carrier.
H2 + 2Z1 ® 2Z1H 1
Z1H + Zn ® Z1+ZnH2
6ZnH + CO® CH4 + H2O + Zn 3
4ZnH + SO2® 2H2O + 4Zn + S4
4ZnH + 2 NO ® N2 + 2H2O + 4 Zn5
8ZnH + CO2® CH4 + H2O + 8Zn 6
The reaction easily runs with F2 and Cl2:
2ZnH + F2 ® 2HF + 2Zn7
2ZnH + Cl2 ® 2HCl + 2Zn 8
The received HF and HCl in the room temperature react with the carbonates and form the corresponding salts. Therefore takes place complete degasation of poisonous gases.
For the verification this thoughts we made the following experiments. The method of experiments was analog with the works [82],[90].
The reactor is the cylinder from pirex glass and has 1,8 cm. diameter. That was situated in the electrical furnacein vertical form. In the central part of reactor , on the pirex glass pear situated the activator of hydrogen (on SiO2 Cu is sat). The size of particles composed 0,2 - 0,3 millimeter. In the different experiences on the activecomponent we put the layers of inert components ( SiO2 + Al2O3 ). The diameter of particles did not surpass 0,2 millimeter.
As we described in work [91] hydrogen is given from below through the zone of active center to a reaction zone. The speed of hydrogen's given were 14 -20 cm3/minute. That was given at the atmospheric pressure. CO is given directly in the reaction zone with partial pressure, equal 15 tor by mobile capillary. For correct definition the temperature of reaction in the central part of reactor was put the mobile slender thermocouple from qromel- alumel d = 0,1 mm, the head of which was not outdo the sizes of particles. Analysis of products are done by gas-liquid qromatografe.
The experiences showed, that in the absence of active componentthere is not any turning of CO in the interval 373 - 673 OK. At the presence of active component the turning of CO it observed even at the temperature of 370 OK. The fundamental products are CH4 and H2O, and the high hydrocarbons and alcohols are raised by traces.
In the Figure 78are given the experimental dates about outlay of CO and formation of CH4 depending on temperature.
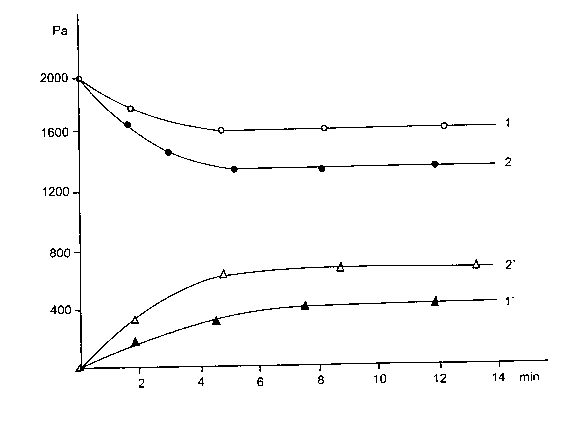
Figure 78. Outlay of CO ( curves 1 and 2 ) and formation of CH4 ( curves 1` and 2` ). 1, 1` -T = 473 OK, 2, 2` - T = 520 OK.
The outlay of CO at 473 OK reachs to 21 %, and at 520OK to 30%. Even at room temperature there is outlay of CO ( 10-1 tor or 13,3 Pa ) during one day ( Figure 79 ). That is the exacttemperature of caves.

Figure 79. Dependence of CO formation from temperature.
With the rising of temperature the percentage of exchangeand the speed grows.Atfirst and atsecond cases the presence vapor of water has positive influence on the process. That influence can explain from different points of view. In [90] was showed, that the hydrogen atoms run badly on the surfaces, onwhich are adsorbed the water molecules. We think, that the molecules of water play the role of bridge, which facilitate passage of the active hydrogen's atoms from the particles of activatorto surface of taking of inert carrier.
So on the walls of caves there are the activatorsof hydrogen and the taking of inert carrierby developed surface. So the run of the reactions ( 1 - 8 ) becomes very probable. The presence of water's vapor raises the speed of that reactions ( in the caves moisture of air approaches to 100 % ).
So on the walls of caves take place the natural processes of hydrogenation cleaning, when the several poisonous gases ( CO, H2S, SO2, F2, Cl2 and others) turn into nonpoisonous gases. Therefore owing to hydrogen's Spilover phenomena the caves acquire important ecological meaning.