It was shown [106],[109] that peracetic and perpropionic acids as well as methyl hydroperoxide decompose in low temperatures on different surfaces of a reactor, as well as on the platinum net [109] by the radical mechanism with moving the radicals to the volume. Investigations showed, that active particles, registered in the volume in each case, are peroxide radicals like RO2. In this part of work the data, received while investigating kinetics of law temperature decomposition of performic acid on the platinum net surface, which brings to formation of appreciable quantity of HO2 radicals unlike the radicals of RO2 type, which had been discovered earlier during decomposition of the organic peroxides, mentioned above, are presented. The performic acid was synthesizing by the method [104]. The experimental methodology and the way of peroxide provision is like one that was described [109] ( Figure 85 ).
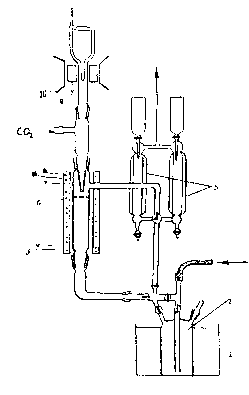
Figure 85. HCO3H catalytic radical decomposition reactor.
Investigations were hold under different pressures: law and atmospheric. In the last case in the presence of the carrier - nitrogen. The experiments showed, that under 280 OC and pressure 0,18 - 2 tor in the process of catalysis decomposition of performic acid on the platinum net, radicals HO2 are transfered in the volume, instead of expected HCOO radicals [110]. The spectrum of elementary process in slow gasophase reactions of HO2 radicals is given in Figure 86.
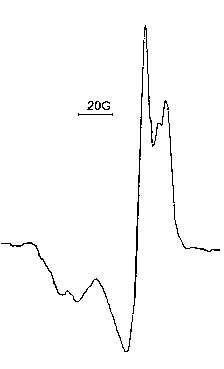
Figure 86. HO2 radical EPR spectrum.
During one minute of the duration of the experiment it was managed to accumulate from 5.1014 up to 5.1015 HO2 radicals in freezing junction, depending on the starting pressure. The intensity of a signal changed depending on the platinum net temperature. With temperature decrease the quantity of radicals, transfered to the volume and accumulated in the freezing junction, decreased perceptibly. But even in 28 OC the decomposition took place and it was possible to register radicals. As it was already said, performoc acid decomposition in gas phase proceed mainly with CO2 formation. CO2 and HO2 radicals formation, probably, is connected with the following elementary processes, proceeding on the platinum surface:
HCO3Hads ® HCOOads + OHads.
HCOOads ® Hads + CO2ads.
Hads + O2ads® HO2.
By analogy with radical decomposition of organic peroxides [107],[109], it is possible to assume, that hydroperoxide radicals occur in interaction H atoms with adsorbed on the platinum oxygen. In order to clarify the probability of HO2 radicals formation not on the catalyzer surface, but on the cold shoot of Duar in interaction desorbred H atoms with molecular oxygen [111] we made special experiments.
It is known, that H atoms recombine with sufficiently higher than HO2 radicals speed on the surface of a grass tube, which leads to the freezing junction from the reactor. Earlier, it was established [112], that lengthening of the way, from the zone of atom or radical formation to the freezing junction, decreases the quantity of H atoms and, correspondingly, HO2 radical formation on the cold surface sharply, while multiple lengthening of the way for HO2 radicals doesn't matter much to signal intensity of elementary processes in slow gasophase reactions. Taking this circumstance into consideration, when elementary processes in slow gasophase reactions signal of high intensity radicals exist, corresponding ~1015 particle/ cm3 the way was lengthened from 35 to 105 cm from the reactor to the shoot of Duar. It occurs, that such lengthening of the way doesn't decrease the intensity of fixed signal of HO2 radicals. So, the experiments testified, that HO2 radicals, frozen on the cold shoot of Duar pass from the catalyzes surface to the volume immediately.
In 150 OC decrease of pressure from 0,28 to 0,18 tor relative output of HO2 radicals in account of starting peroxide increases two times. It means that the less the time of contact ( tcont ). of gas stream, containing performic acid with the surface of platinum is, the more radicals output is. If to take into account, that quantity of starting and a part of decomposed acid decreases, then in reality, correlation of the quantity of accumulated radicals on the frozen shoot of Duar to the quantity of decomposed peroxide will be more. Increase of radicals output into the volume with the decrease of time of contact ( tcont. )obviously, is connected with the decrease of their heterogeneous recombination thanks to the backward diffusion of the active particles to the surface of platinum.
In all experiments in low temperatures the quantity of used peroxide happened to be lower than sensitivity of applied method of analysis kinetic regularity of performic acid utilization and radical accumulation depending on temperature and its concentration in mixture were studied under atmospheric pressure in a nitrogen stream, so as to say, in conditions, in CH3CO3H on the platinum net was investigated [110].
Concentration HCO3H in nitrogen stream was 0,1; 0,15 and 0,22%. The experiment showed that performic acid decomposition proceeds with less speed than peracetic acid decomposition on the platinum net [109].
In Figure 87, kinetic curves of performic acid utilization, got in 305, 330, 345 OC are given. The content of peracid is 0,22%.
Figure 87. HCO3H decomposition kinetics in different temperatures: 1 - 305, 2 - 330,3 - 345 OC, [HCO3H]O = 0,22 %, 1 and 1`` - in T = 305 OC and [HCO3H] = 0,10 and 0,15 % correspondingly.
As it is seen from the Figure 87, with tcont. increase, the quantity of used peroxide increases. The higher the temperature of the experiment is, the more HCO3H conversion is. The activation of energy estimated by starting speeds of peracid utilization happen to be equal to 10 ± 2 kkal/mol. Low meaning of energy activation testified about heterogenous nature of HCO3H decomposition on the platinum.
In order to clarify the influence of starting concentration of performic acid to the kinetics of decomposition varied its content in a nitrogen stream. The experiments proceeded in 305 OC and [HCO3H]O =0,1; 0,15 and 0,22 %. It was established, that a part of decomposed peroxide in given temperature doesn't depend on its starting concentration. It is illustrated well by the curve 1 in Figure 87, which describes the kinetics of peroxide utilization along the ordinate axis stretch concentration of performic acid is given. As is seen, points, corresponding to different starting concentrations of peroxide are on the same curve, so, in such short time of contact, in the conditions of given experiment HCO3H decomposition proceeds by the first order.
In Figure 88 the kinetic curves of HO2 radicals accumulation, got while estimating decomposition of performic acid in nitrogen stream under atmospheric pressure in T = 305, 330, 345 OC and [HCO3H]O = 0,22 %.

Figure 88. Kinetic curves of HO2 radicals accumulation under the influence of temperature and HCO3H concentration: 1, 2, 3 - in T = 305, 330 and 345 OC and [HCO3H]O = 0,22 %. 1` and 1`` - in T = 305 OC and [HCO3H] = 0,15 and 0,1 %.
As it is seen from the Figure 88, with the increase of contact time ( tcont. ) the radicals output increases, reaches maximum, then it falls down with the use of starting peroxide. Maximum concentration of HO2 radicals, accumulated in freezing junction increases with the increase of temperature. The kinetic of radical accumulation in T = 305 OC and [HCO3H]O = 0,1; 0,15 and 0,22 % is described by 1, 1`, 1`` curves. Radicals output increases with the increase of the starting concentration of peroxide. As in the work [109] the maximum concentration of radicals is proportional to peroxide concentration.
In order to clarify the question, whether existing in nitrogen imprints of oxygen are enough ( 0,5 % ) for quantitative formation of HO2 radicals, the following experiments were done. In time of contact 5.10-4 sec, and T = 350 OC in nitrogen stream, containing 0,18 % peroxide, oxygen was delivered in 0,8 - 3 % quantity. The experiments showed, that addition of oxygen didn't influence either value of signal of elementary processes in slow gasophase reactions, or quantity of used acid.
The main kinetic regularities of decomposition of performic acid and peracetic acid on the platinum net happen to be approximately the same. But it is necessary to pay attention to that circumstance, that in such big conversions of performic acid ( Figure 87 ), relatively little in comparison with data of radical decomposition of CH3CO3H [109] quantities of peroxide radicals pass to the volume. So one may come to the conclusion, that in the case with performic acid relatively not a big part of decomposed peroxide is realized in the shape of HO2 radicals and H atoms and HO2 radicals occurred on the surface of the catalyzer recombine, giving H2O. What deals with feasible origin of hydrogen, taking the sensitivity of used chromatographic method of analysis into consideration, one may conclude, that quantity of it is less at least 0,22 % in gas.
In a number of cases the reaction of heterogeneous decomposition of performic acid can be recommended as the source of HO2 radicals.
So, not only the role of HCO3H in anaerobic reactions, as significant circuit of methane and carbon dioxide gas generating process, is revealed, but also an opportunity of existence of whole number of reactions, in which HO2 radicals occurring of HCO3H decomposition on the hard surface ( walls of caves, stalactites, stalagmites and hard surfaces in general ) participate. This is a new sphere of investigations, which demands deeper radical level researches or the reaction, proceeding in caves.